Abstract
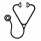
Introduction
Recent work has questioned the value of surveillance imaging in several non-Hodgkin lymphoma subtypes (Cohen 2014). While scheduled surveillance imaging of patients with follicular lymphoma (FL) who achieved first remission is a common practice, no consensus exists on the type, duration, frequency, or need for routine imaging studies as part of follow-up care. We examined patterns of surveillance imaging, assessed the impact of surveillance imaging on relapse detection, and evaluated outcomes for patients with FL based on method of relapse detection.
Methods
All FL patients evaluated at Emory with documented response to front-line induction and available follow-up data were included. We assessed each patient for treatment response, presence of relapse, and method of relapse detection in addition to baseline variables of interest. Patients were classified as having a relapse detected clinically (via the presence of symptoms, abnormal exam, or lab findings) or by routine surveillance. We described progression-free survival (PFS), defined as the time from the date of diagnosis to relapse, death without relapse, or last known follow-up, and overall survival (OS). OS was defined as the time from the date of diagnosis to the date of death or last known follow-up and also from the date of relapse to examine the impact of the method of relapse detection. We used Kaplan Meier method, log-rank test, and Cox proportional hazards model to compare survival outcomes between clinical and radiographic methods of initial relapse detection. Available individual computed tomography (CT) and positron emission tomography (PET/CT) scan results were reviewed, and we determined the positive and negative predictive values (PPV, NPV), sensitivity, and specificity.
Results
Of the 148 included patients, 82 (55%) were female and 66 (45%) were male. Median age at diagnosis was 57 years (22-83), and 120 patients (81%) had grade 1-2 disease. Most patients (n=108, 71%) had stage 3-4 disease. Of 132 patients with available data, 61 (46%) had a high-risk FLIPI score (≥3). The median observation period for the cohort was 70.4 months (range: 7-308).
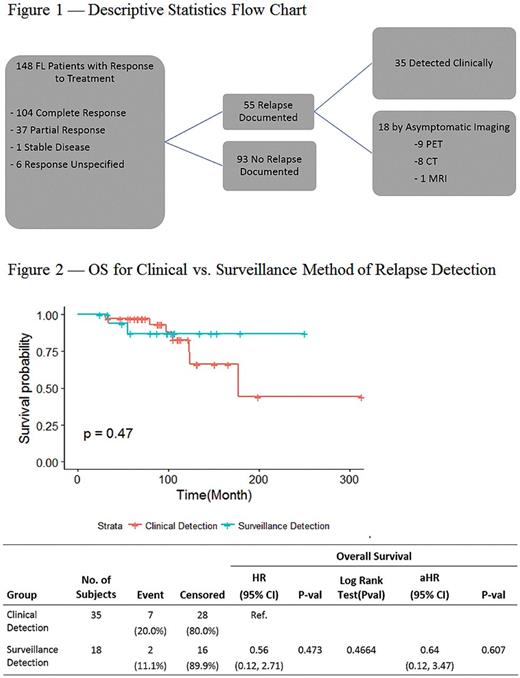
Fifty-five patients (37%) experienced a confirmed relapse during the observation period. The median time from diagnosis to first confirmed relapse for those who relapsed was 43.7 months (6-168). The median follow-up for those who did not relapse was 51.1 months (7-220). Of the 55 relapses, 35 (64%) were detected clinically, and 18 (33%) asymptomatic cases were detected on routine surveillance imaging (Figure 1). Two patients (3%) did not have the method of relapse detection documented. There was no significant difference in OS from diagnosis between those whose relapse was detected clinically vs. those whose relapse was detected by routine surveillance imaging (HR=0.56 [0.12, 2.71], p=0.47; Figure 2). Similarly, there was no significant difference in OS from the date of relapse (HR= 0.46 [0.09, 2.22], p= 0.33).
All 656 individual imaging studies from 130 patients who underwent at least part of their surveillance at Emory were evaluated. Of these scans, 443(67%) were CT scans, 188 (29%) were PET/CT, 24 (4%) were MRI, and 1 was a mammogram. Seventy-one (11%) scans were performed due to a clinical concern, and 32 of these scans (45%) resulted in a confirmation of relapse. 584 scans were conducted as surveillance for asymptomatic patients, and 68 (12%) of these scans were concerning for relapse. Twenty-two (32%) of the 68 concerning surveillance scans ultimately resulted in confirmed relapse. As a result, 22 of 584 surveillance scans (3.8%) led to identification of a confirmed FL relapse. Overall, the surveillance scans had a 92% specificity, a 91% sensitivity, a NPV of 99.6%, and a PPV of 32% across all imaging modalities. CT scans had a higher specificity than PET/CT scans (95% vs 83%), but had a lower sensitivity (89% vs 100%).
Conclusion
Most cases of relapse in FL are detected based on clinical signs and symptoms. No significant difference in OS was found between the cases where relapse was first suggested on surveillance compared to those detected clinically. Only 1 out of every 25 routine surveillance scans led to a diagnosis of relapse. While routine surveillance after first-line induction in FL is a common practice, these findings suggest they may have limited utility. Larger studies across multiple centers are recommended to further confirm the conclusion of this study.
Calzada: Seattle Genetics: Research Funding. Flowers: Educational Concepts: Research Funding; Abbvie: Consultancy, Research Funding; Seattle Genetics: Consultancy; TG Therapeutics: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; OptumRx: Consultancy; Gilead: Consultancy; Spectrum: Consultancy; Prime Oncology: Research Funding; Celgene: Consultancy, Research Funding; V Foundation: Research Funding; National Cancer Institute: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Burroughs Welcome Fund: Research Funding; Millennium/Takeda: Research Funding; Acerta: Research Funding; Bayer: Consultancy; Infinity: Research Funding; Janssen Pharmaceutical: Research Funding; Onyx: Research Funding; Genentech/Roche: Consultancy, Research Funding; Research to Practice: Research Funding; National Institutes Of Health: Research Funding; Clinical Care Options: Research Funding. Cohen: Bristol Myers Squibb: Research Funding; Bioinvent: Consultancy, Membership on an entity's Board of Directors or advisory committees; Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; LAM Therapeutics, Inc: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takada: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract